zn valence electrons|The Valence Orbital Diagram for Zn: Unraveling the Electron : Pilipinas Mar 23, 2023 Specializing in Video Slots. In common with many other developers, such as the studios behind Gamescale slots, or Simbat slots, most of the games from JILI are video slots.This means they combine the classical elements of slot machines with more innovative game design, such as HD animations, engaging themes, and immersive soundtracks.PCH has various sweepstakes that give you a chance to win up to $1,000,000 dollars! Enter now.
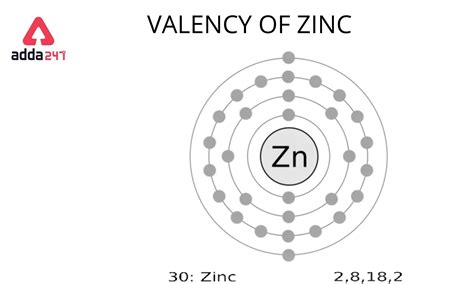
zn valence electrons,Zn – 2e – → Zn 2+. Here, the electron configuration of zinc ion is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. This electron configuration shows that zinc ion (Zn 2+) has three shells and the last shell has eighteen .
Mar 23, 2023 To find the number of valence electrons for Zinc (Zn) we need to look at its electron configuration. This is necessary because Zn is a transition metal (d block element) and we need to take.zn valence electrons Zinc has only 2 valence electrons, both located in the 4s-orbital. This is based on its electron configuration and its ability to lose these electrons to form Zn2+ cation. See the detailed answer and .zn valence electrons The Valence Orbital Diagram for Zn: Unraveling the Electron Zinc has only 2 valence electrons, both located in the 4s-orbital. This is based on its electron configuration and its ability to lose these electrons to form Zn2+ cation. See the detailed answer and .

Find out the valences of the chemical elements, including zinc, from the .
Learn how to determine the number of valence electrons for an element using the periodic table. See patterns, examples, and tips for main group and transition metals. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as .In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell .
The Valence Orbital Diagram for Zn: Unraveling the Electron Periodic table history. Identifiers. List of unique identifiers for Zinc in various chemical registry databases. Zinc is a chemical element of the periodic table with chemical symbol .Learn how to draw and interpret the valence orbital diagram for zinc (Zn), a transition metal with atomic number 30. The diagram shows that Zn has two valence electrons in the .
Le tableau périodique montre que les numéros atomiques du zinc (Zn) sont de 30. Cela signifie que le nombre total d’électrons dans l’atome de zinc est de trente. Les termes « degré d’oxydation » et « .
Overview Electron configuration. The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. Thus, the number of valence electrons that it may have . In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. Valence electrons are outer shell electrons for .The valency of Zn is 2 and the valency of oxygen is also 2. Construct the formula for zinc oxide. Q. Zinc does not show the variable valency as elements of d-block, because: Q. Zn (30) : 1s22s22p63s23p63d104s2.
Zinc ion (Zn 2+) electron configuration. The ground state electron configuration of zinc is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2. The electron configuration shows that the last shell of zinc has two electrons and the d-orbital has a total of ten electrons. Therefore, the valence electrons of zinc are two.Zinc (Zn) Zinc is the 30th element in the periodic table and has a symbol of Zn and atomic number of 30. It has an atomic weight of 65.38 and a mass number of 64. Zinc has thirty protons and thirty-four neutrons in its nucleus, and thirty electrons in four shells. It is located in group twelve, period four and block d of the periodic table.
3.1: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..

The valence electrons (i.e., the electrons in the outermost subshell) have the highest energy.They are particularly important because they are involved in bond formation and determine the chemical properties of an element.Find out more about how the attraction of an electron to a nucleus will change with our effective nuclear charge .
zn valence electrons|The Valence Orbital Diagram for Zn: Unraveling the Electron
PH0 · Zinc (Zn)
PH1 · Valences of the Chemical Elements
PH2 · Valence electrons (video)
PH3 · Valence electron
PH4 · Valence Electrons Chart for All Elements
PH5 · The Valence Orbital Diagram for Zn: Unraveling the Electron Configurati
PH6 · The Valence Orbital Diagram for Zn: Unraveling the Electron
PH7 · How to Find the Valence Electrons for Zinc (Zn)?
PH8 · How to Find the Valence Electrons for Zinc (Zn)
PH9 · How many valence electrons does zinc have?
PH10 · Determine valence electrons using the periodic table